Which Transport Mechanism Can Bring Whole Cells Into a Cell?
5.7: Cell Ship
- Page ID
- 22742
Look at the big windows and drinking glass doors in this firm. Imagine all the light they must let in on a sunny day. Now imagine living in a house that has walls without whatever windows or doors. Zip could enter or leave. Or imagine living in a firm with holes in the walls instead of windows and doors. Things could enter or leave, only you lot couldn't control what came in or went out. Just if a house has walls with windows and doors that tin be opened or closed yous can control what enters or leaves. For example, windows and doors allow you lot to let in calorie-free and the family domestic dog and go on out rain and bugs.
.jpg?revision=1&size=bestfit&width=441&height=285)
Transport Across Membranes
If a cell were a house, the plasma membrane would exist walls with windows and doors. Moving things in and out of the cell is an important office of the plasma membrane. It controls everything that enters and leaves the cell. At that place are 2 basic ways that substances tin cross the plasma membrane: passive transport, which requires no free energy; and active transport, which requires energy. Passive send is explained in this department and Agile transport is explained in the adjacent section, Active Send and Homeostasis. Various types of jail cell ship are summarized in the concept map in Effigy \(\PageIndex{two}\).
Transport Without Free energy
Passive transport occurs when substances cross the plasma membrane without any input of energy from the cell. No free energy is needed because the substances are moving from an area where they have a higher concentration to an area where they have a lower concentration. H2o solutions are very important in biology. When h2o is mixed with other molecules this mixture is called a solution. Water is the solvent and the dissolved substance is the solute. A solution is characterized by the solute. For example, h2o and sugar would exist characterized as a sugar solution. More than the particles of a solute in a given volume, the higher the concentration. The particles of solute always move from an area where it is more concentrated to an area where it is less concentrated. It's a fiddling like a ball rolling downward a hill. Information technology goes past itself without whatsoever input of actress energy.
The different categories of prison cell transport are outlined in Figure \(\PageIndex{2}\). Jail cell transport tin can be classified as follows:
- Passive Send which includes
- Simple Diffusion
- Osmosis
- Facilitated Diffusion
- Active Transport can involve either a pump or a vesicle
- Pump Transport can be
- primary
- secondary
- Vesicle Transport can involve
- Exocytosis
- Endocytosis which includes
- Pinocytosis
- Phagocytosis
- Receptor-Mediated Endocytosis
- Pump Transport can be
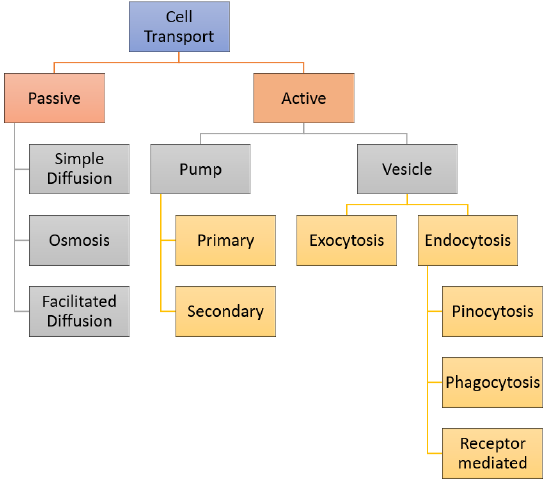
Figure \(\PageIndex{two}\): The Prison cell Transport Concept Map illustrates various types of cell transports that happen at the plasma membrane
Unproblematic Diffusion
Diffusion Although you may not know what diffusion is, yous have experienced the process. Tin can you lot remember walking into the front door of your dwelling house and smelling a pleasant smell coming from the kitchen? Information technology was the diffusion of particles from the kitchen to the front end door of the house that allowed you to observe the odors. Improvidence is defined as the internet movement of particles from an area of greater concentration to an area of lesser concentration.

The molecules in a gas, a liquid, or a solid are in constant movement due to their kinetic free energy. Molecules are in constant move and collide with each other. These collisions cause the molecules to motion in random directions. Over time, however, more molecules will exist propelled into the less concentrated expanse. Thus, the net move of molecules is always from more tightly packed areas to less tightly packed areas. Many things can diffuse. Odors lengthened through the air, salt diffuses through h2o and nutrients diffuse from the blood to the torso tissues. This spread of particles through the random motility from an area of loftier concentration to an area of lower concentration is known as diffusion. This unequal distribution of molecules is called a concentration gradient. Once the molecules get uniformly distributed, a dynamic equilibrium exists. The equilibrium is said to be dynamic because molecules continue to move, simply despite this change, there is no cyberspace change in concentration over time. Both living and nonliving systems experience the process of diffusion. In living systems, improvidence is responsible for the movement of a large number of substances, such as gases and small uncharged molecules, into and out of cells.
Osmosis
Osmosis is a specific type of diffusion; it is the passage of water from a region of loftier water concentration through a semi-permeable membrane to a region of low water concentration. Water moves in or out of a cell until its concentration is the same on both sides of the plasma membrane.
Semi-permeable membranes are very thin layers of material that allow some things to laissez passer through them just preclude other things from passing through. Cell membranes are an example of semi-permeable membranes. Cell membranes allow small molecules such equally oxygen, water carbon dioxide, and oxygen to pass through but do non allow larger molecules similar glucose, sucrose, proteins, and starch to enter the cell straight.
The classic case used to demonstrate osmosis and osmotic pressure is to immerse cells into sugar solutions of diverse concentrations. There are three possible relationships that cells tin can run into when placed into a sugar solution. Effigy \(\PageIndex{4}\) shows what happens in osmosis through the semi-permeable membrane of the cells.
- The concentration of solute in the solution can be greater than the concentration of solute in the cells. This cell is described as being in a hypertonic solution (hyper = greater than normal). The net flow or water will be out of the cell.
- The concentration of solute in the solution tin can be equal to the concentration of solute in cells. In this situation, the prison cell is in an isotonic solution (iso = equal or the aforementioned equally normal). The amount of water inbound the cell is the same as the amount leaving the cell.
- The concentration of solute in the solution can exist less than the concentration of solute in the cells. This jail cell is in a hypotonic solution (hypo = less than normal). The net flow of water will be into the prison cell.
Figure \(\PageIndex{5}\) demonstrates the specific outcomes of osmosis in ruby-red blood cells.
- Hypertonic solution. The red blood cell will appear to shrink every bit the water flows out of the cell and into the surrounding environment.
- Isotonic solution. The scarlet blood prison cell volition retain its normal shape in this environs as the amount of water entering the prison cell is the same as the amount leaving the cell.
- Hypotonic solution. The red blood cell in this environment volition become visibly swollen and potentially rupture as h2o rushes into the jail cell.
Facilitated Diffusion
Water and many other substances cannot just diffuse across a membrane. Hydrophilic molecules, charged ions, and relatively big molecules such as glucose all need aid with diffusion. The help comes from special proteins in the membrane known as transport proteins. Diffusion with the help of transport proteins is chosen facilitated improvidence. At that place are several types of transport proteins, including channel proteins and carrier proteins (Effigy \(\PageIndex{6}\))
- Aqueduct proteins form pores, or tiny holes, in the membrane. This allows water molecules and small ions to pass through the membrane without coming into contact with the hydrophobic tails of the lipid molecules in the interior of the membrane.
- Carrier proteins bind with specific ions or molecules, and in doing and then, they alter shape. As carrier proteins change shape, they carry the ions or molecules across the membrane.
Review
- What is the main difference between passive and active transport?
- Summarize 3 different ways that passive transport can occur, and give an example of a substance that is transported in each way.
- Explain how transport across the plasma membrane is related to the homeostasis of the cell.
- Why tin generally merely very small, hydrophobic molecules across the cell membrane by unproblematic diffusion?
- Explain how facilitated diffusion assists in osmosis in cells. Be sure to define osmosis and facilitated diffusion in your answer.
- Imagine a hypothetical cell with a higher concentration of glucose within the cell than exterior. Answer the following questions about this jail cell, assuming all transport across the membrane is passive, not active.
- Tin can the glucose simply lengthened across the cell membrane? Why or why not?
- Assuming that at that place are glucose transport proteins in the cell membrane, which manner would glucose flow – into or out of the cell? Explicate your answer.
- If the concentration of glucose was equal inside and outside of the cell, exercise y'all recall in that location would be a net flow of glucose beyond the cell membrane in one direction or the other? Explain your respond.
- What are the similarities and differences between channel proteins and carrier proteins?
- True or Simulated. Simply active send, not passive send, involves transport proteins.
- Truthful or Imitation. Oxygen and carbon dioxide tin squeeze betwixt the lipid molecules in the plasma membrane.
- True or Simulated. Ions easily diffuse across the cell membrane by simple diffusion.
- Decision-making what enters and leaves the cell is an important role of the:
- nucleus
- vesicle
- plasma membrane
- Golgi appliance
Explore More
Source: https://bio.libretexts.org/Bookshelves/Human_Biology/Book%3A_Human_Biology_(Wakim_and_Grewal)/05%3A_Cells/5.07%3A_Cell_Transport

0 Response to "Which Transport Mechanism Can Bring Whole Cells Into a Cell?"
Post a Comment